Looking for new antiinflamMAtory analgesic compounds
In the lab we study the potential therapeutic action of different neuropeptides. These compounds are produced by our own body in many different circumstances in response to homeostasis alteration. In this work we focus in the study of analgesic properties of one peptide that we previously described as a pontent antiinflamatory: cortistatin. Similar to somatostatin, CST is able to bind to the same receptors but has unique functions as sleep promoting or decreasing locomotor activity.
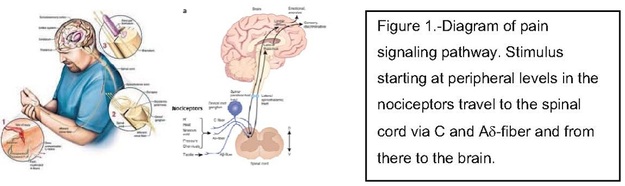
Chronic pain represents a frequent and poorly understood medical problem in patients with osteoarthritis and rheumatoid arthritis. Following joint injury and local inflammation, release of inflammatory cytokines, chemokines, protons, vasoactive amines and prostaglandins induces the excitation of peripheral terminals (C-fibers and Aδ-axons) of nociceptive neurons. This response results in the release of aminoacids (glutamate/aspartate) and neuropeptides (substance P and calcitonin gene-related peptide/CGRP) by the central terminal of nociceptor at the spinal level, the activation of spinal second order neurons and the subsequent cascade of ascending nociceptive pathways to the brain. An analgesic, also called “pain killer”, is a drug able to inhibit this signaling. It can acts at different levels , central or periphery.
In the present work we investigate the possible analgesic properties of the anti-inflammatory neuropeptide cortistatin in chronic pain evoked by joint inflammation.
We assessed the effects of cortistatin administered centrally, peripherally and systemically in thermal and mechanical hyperalgesia evoked in mice by intraarticular infusion of carrageenan/kaolin into knee joints. The effect of cortistatin in the production of nociceptive mediators (CGRP) and the activation of pain signaling (phosphorylated-ERK) was assayed in dorsal root ganglia cultures and in inflammatory pain models. We finally assayed the effects of noxious/inflammatory stimuli in the production of cortistatin by the peripheral nociceptive system in vitro and in vivo. We found that cortistatin is expressed in the murine nociceptive system by peptidergic nociceptors and that endogenous cortistatin participates in tuning pain sensitization especially in pathologic conditions. We demonstrate that cortistatin acts peripherally and centrally reducing tactile allodynia and heat hyperalgesia in arthritis via mechanisms that are independent of its anti-inflammatory action and that involve direct action on nociceptive neurons and regulation of central sensitization. Therefore, we propose that the nociceptive system responds to noxious stimuli producing cortistatin as a natural analgesic factor to further counterbalance the action of nociceptive mediators.
Cortistatin emerges as an anti-inflammatory factor with potent analgesic effects that offers a new approach to pain therapy in pathologic inflammatory states, including osteoarthritis and rheumatoid arthritis.
